By Brendan Pierson
(Reuters) -A Delaware judge has allowed more than 70,000 lawsuits over discontinued heartburn drug Zantac to go forward, ruling that expert witnesses can testify in court that the drug may cause cancer.
The ruling on Friday by Judge Vivian Medinilla of the Delaware Superior Court in Wilmington is a setback for former Zantac makers GSK (LON:GSK), Pfizer (NYSE:PFE), Sanofi (EPA:SASY) and Boehringer Ingelheim, which had argued that the expert witnesses' opinions lacked scientific support.
Medinilla wrote that the strength of each sides' scientific arguments should be decided by juries.
"Delaware courts are loath to step into the heart of technical debate between opposing scientists," she said.
“This moves us one step closer to justice for our clients,” Brent Wisner, one of the plaintiffs' lead lawyers, said in a statement on Saturday.
GSK, Pfizer and Sanofi said in separate statements that they disagreed with the decision and would appeal. They said there was no reliable evidence showing Zantac caused cancer. A spokesperson for Boehringer Ingelheim did not immediately respond to a request for comment.
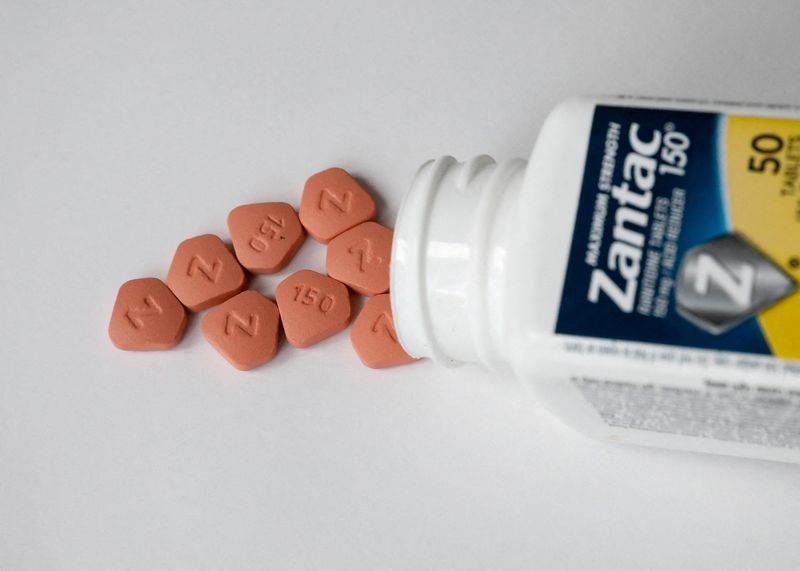
In 2019, some manufacturers and pharmacies halted Zantac sales after a chemical called NDMA, which is known to cause cancer, was detected in some pills. Some tests showed that Zantac's active ingredient, ranitidine, could degrade into NDMA over time or when exposed to heat.
Lawsuits began piling up from people who said they developed cancer after taking Zantac. Plaintiffs said the companies knew, or should have known, that ranitidine posed a cancer risk and that they failed to warn consumers.
The U.S. Food and Drug Administration asked manufacturers to pull the drug off the market in 2020. The drugmakers have maintained that there is no evidence Zantac exposed users to harmful levels of NDMA.
Medinilla is presiding over the majority of nearly 80,000 cases still pending in the United States over Zantac, which was once the world's top-selling drug.
In addition to the cases in Delaware, the drugmakers are facing about 4,000 claims in California state court and about 2,000 in various other state courts around the country.
Last month a jury in Chicago rejected an Illinois woman’s claim that Zantac caused her colon cancer, handing GSK and Boehringer Ingelheim a victory in the first case to go to trial.
The drugmakers notched a significant win in 2022, when another judge dismissed about 50,000 lawsuits making similar claims that had been consolidated in federal court in Florida.
That judge concluded that the opinions of the plaintiffs' expert witnesses that Zantac can cause cancer were not supported by sound science. Plaintiffs are appealing that ruling, which concerns different experts from those in the Delaware case.
1988 and one of the first-ever drugs to top $1 billion in annual sales. Originally marketed by a forerunner of GSK, it was later sold successively to Pfizer, Boehringer and finally to Sanofi.